
The polypeptide chains of fibrous proteins such as collagen are aligned along an axis, have repeating elements, and are extensively linked to each other through hydrogen and covalent bonds. Assembly of globular polypeptide subunits into a multisubunit complex can provide the opportunity for cooperative binding of ligands (e.g., O 2 binding to hemoglobin), form binding sites for complex molecules (e.g., antigen binding to immunoglobulin), and increase stability of the protein. Folds are defined by their similarity in a number of different proteins. Within a domain, a combination of secondary structural elements forms a fold, such as the nucleotide-binding fold or an actin fold. Multiple domains can be linked together to form a functional protein. The tertiary structure of a globular protein can be made up of structural domains, regions of structure that fold independently. Some proteins exhibit quaternary structure, the combination of two or more subunits, each composed of a polypeptide chain.įIGURE 7.1 Levels of structure in a protein.ĭomains and Folds. In globular proteins such as myoglobin, the tertiary structure generally forms a densely packed hydrophobic core with polar amino acid side chains on the outside. The tertiary structure involves folding of the secondary structural elements into an overall three-dimensional conformation.

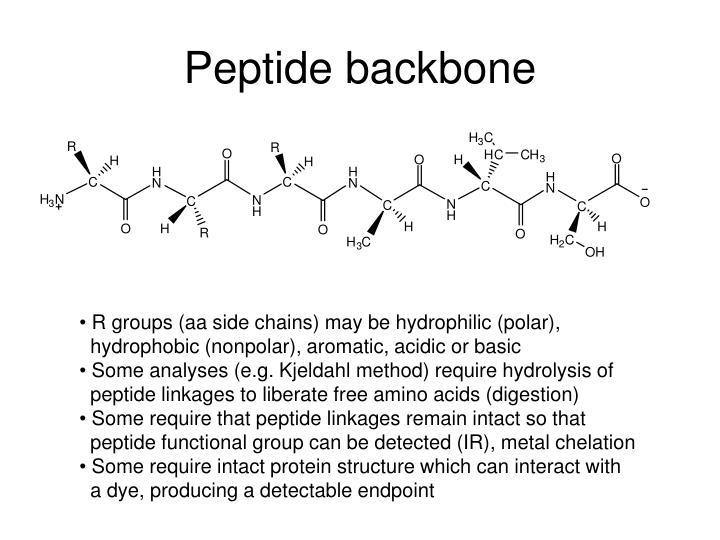
The rigidity of the peptide backbone determines the types of secondary structure that can occur. Secondary structure consists of local regions of polypeptide chains formed into structures that are stabilized by a repeating pattern of hydrogen bonds, such as the regular structures called α-helices and β-sheets. The primary structure of a protein is the linear sequence of amino acids in the polypeptide chain. Protein structure is described in terms of four different levels: primary, secondary, tertiary, and quaternary ( Fig. Even in sickle cell anemia, the mutation in hemoglobin principally affects the quaternary structure of hemoglobin and its solubility, and not its ability to bind oxygen. Prion diseases result from misfolding and aggregation of a normal cellular protein.
Alzheimer disease and familial amyloid polyneuropathy are neurodegenerative diseases characterized by the deposition of amyloid. In amyloidosis/AL, immunoglobulin chains form an insoluble protein aggregate called amyloid in organs and tissues. They also can be caused by conformational changes in proteins that affect their solubility and degradability. Diseases can be caused by changes in protein structure that affect the protein’s ability to bind other molecules and carry out its function.


 0 kommentar(er)
0 kommentar(er)
